Microgravity manufactured zeolites (microporous minerals) with tiny cavities for hydrogen gas storage and more.
Updated: 2024-02-24
Created: 2018-11-01
Status
Basic experiments performed in space, but have not found a path to cost-effective use in terrestial industries. The zeolite mass ratio to storable hydrogen amount seems poor, because of space transport costs, but good sources are lacking.
Applications
- Zeolites to store hydrogen gas in vechicles and stationary facilities.
- Zeolites for catalysts and absorbents.
- Zeolites for more efficient petroleum (chemical) processing.
- Zeolites for “green” household products, environment monitoring and control, zeoponic materials, photocatalysts.3
Why & Solution
Hydrogen will be an excellent energy source for the future. It can be combusted with oxygen to run automobiles without polluting the air. This can be applies to airplanes, boats, buses, trains and any typoe of internal combustion engine. The future of Earth's climate may depend on the use of hydrogen instead of carbon (petroleum) for transportation.4
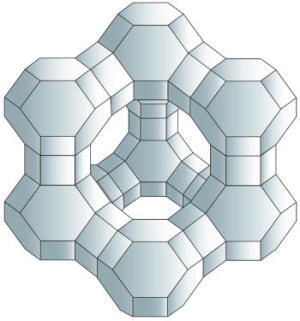
Zeolites are naturally occuring stones that have tiny cavities of 2-8 microns in size. These cavities will absorb and hold gases (like hydrogen), and can be used as tiny lightweight storage containers. The problem is that the cavities in the zeolites are too small. They need to be grown in space so that the zero gravity will allow for larger cavities for more hydrogen containment.4
Zeolites are used for many types of chemical processing. Because of their unique honeycomb features they can act as tiny sieves that allow the filtering of one chemical from another. The cracking of oil into gasoline using zeolites is a standard process. Better efficency through space grown zeolites would allow for getting more gasoline from less raw crude, and this could lesson dependency on foreign oil. Because of over 40% of a barrel of crude is used for gasoline, a 1% increase in gasoline production from a given barrel of oil would result in benefits of hundreds of millions of dollars per year.4
Zeolite means boiling stone and the term refers to over 200 different minerals that have all kinds of interesting uses, from water softeners and cat litter to animal food and industrial catalysts. One of the biggest everyday uses for zeolites is in water softeners and water filters. In ion-exchange water softeners, for example, hard water (rich in calcium and magnesium ions) is piped through a column filled with sodium-containing zeolites. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in sodium. Many everyday laundry and dishwasher detergents contain zeolites to remove calcium and magnesium and soften water so they work more effectively. Two other very common, everyday uses of zeolites are in odor control and pet litter; in both, the porous crystalline structure of the zeolites helps by trapping unwanted liquids and odor molecules.1
Larger, better crystals. Microgravity enables the growth of large, uniform, high-quality/zeotypes ETS titanosilicate crystals; reduced defect concentrations and types; attunement of chemical formulation to control nucleation, growth and chemical control/functionalization.3
A microgravity environment provides quite different conditions for zeolite crystal growth, including elimination of settling and convection, reduced collision breeding, and enhanced diffusion-limited growth. It was hypothesized that the microgravity environment in low Earth orbit would minimize sedimentation, leading to the possibility of producing large zeolite crystal by allowing crystals a longer residence time in a high concentration nutrient field. The microgravity environment should lead to zeolite crystal having a high degree of crystalline perfection with controlled defect concentration and location. With this intention, zeolite crystallization has been carried out under microgravity conditions on Space Shuttle missions. The diffrent nucleation and growth history of zeolite crystal in microgravity resulted in improved crystal morphology and an increase in crystal size, but they frequently led to different microporosity as well as the uniformity and symmetry of the orbit-grown crystal were attributed to a higher degree of crystalline perfection. The synthesis of high-silica ZSM-5 in microgravimetry resulted in crystals with intergrown and thus imperfect disk morphology up to 70 um in diameter. However, very similar space-grown ZSM-5 materials showed a reduction in external surface area and activity, which is interesting for shape-selective catalysis.2 5
- The ZCG investigations examined how subtle changes in the chemical formulation affected nucleation and growth of zeolite crystals. The microgravity environment allowed researchers to grow higher-quality crystals. These crystals have a number of useful commercial applications as catalysts and absorbents.
- This investigation examined how microgravity affects formation and growth of zeolite/zeotype crystals. The three-dimensional structure of a zeolite crystal allows it to act as a molecular sieve to selectively filter certain chemicals in applications such as petroleum processing.
- ZCG furnace provided larger zeolite crystals with fewer defects to characterize their structures, establish structure property relationship, and better understand their sorption, ion exchange, and catalytic performance in order to design new and better materials for use in traditional as well as new applications.
Earthly Solution Risk
Exists, but difficult to estimate at this time.